RA is a vicious cycle, but ORENCIA can help break the cycle early by targeting the T cells directly
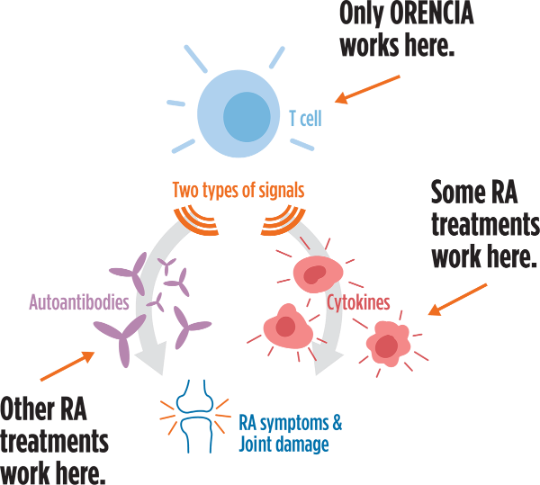
T cells are important because they tell your immune system to react when there is an outside invader in your body. In RA, your own cells are seen as invaders. Once identified, your body activates your T cells to attack your cells, resulting in inflammation in your body.
ORENCIA disrupts T cell activation and prevents sending 2 kinds of signals (autoantibodies and cytokines) that act in different ways to trigger a response in your joints. It is not known how blocking T cells helps with moderate to severe RA symptoms.
Some RA treatments focus only on cytokines or autoantibodies. If you are taking ORENCIA, you know you are being treated with a different approach.